news
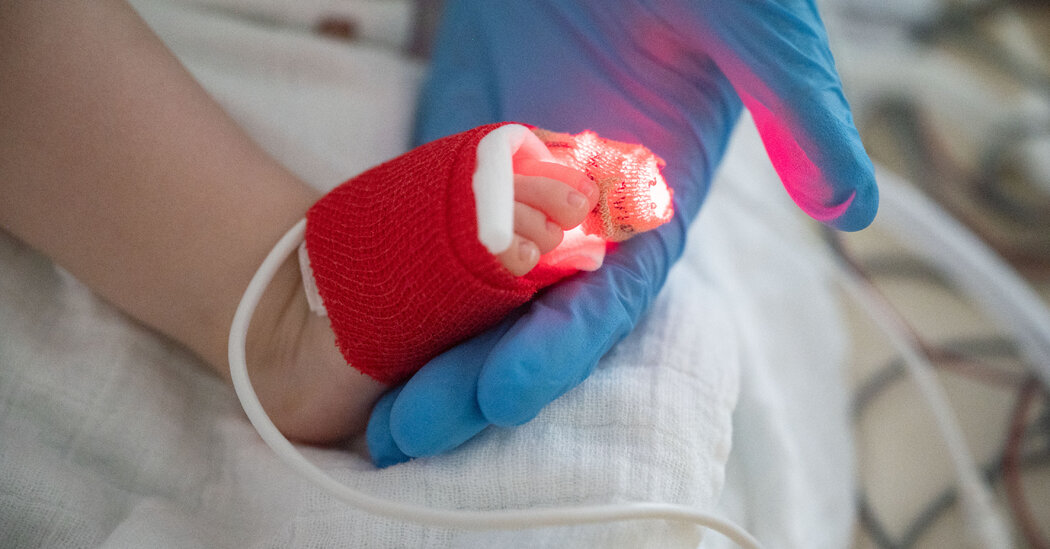
An advisory panel to the U.S. Food and Drug Administration has recommended approval of a monoclonal antibody vaccine designed to protect infants and vulnerable young children from a potentially fatal pathogen, respiratory syncytial virus, or RSV.
The treatment, called Beyfortus by its developers Sanofi and AstraZeneca, will be the second such treatment the FDA has allowed to give young children to prevent RSV, the leading killer of infants and young children worldwide. A similar treatment, approved more than 20 years ago, is given in multiple doses and is approved only for high-risk infants.
The 21-member panel voted unanimously in favor of the treatment for infants born in or entering their first RSV season. Advisers voted 19-2 to vaccinate children younger than 24 months who are still vulnerable to serious illness.
Why it matters: RSV is a worldwide killer of infants.
Although many people think of this common virus as the common cold, it can be serious for young babies and the elderly. According to the Centers for Disease Control and Prevention, as many as 80,000 children under the age of 5 are hospitalized and as many as 300 die each year from the virus. RSV played a role in filling children’s hospitals during this winter’s “triple pandemic,” which also included influenza and Covid-19.
Among adults 65 and older, as many as 160,000 people were hospitalized with RSV and about 10,000 died. Vaccines for older adults were also recently approved.
Background: The security of the footage will be monitored.
In studies provided to the FDA by the drugmaker, more than 3,200 infants were given antibody shots, and one found that after six months it was 79 percent effective against very severe forms of RSV that required medical attention.
A separate agency group recommended approval of a maternal RSV vaccine, also under review. Some advisers have raised concerns about data for this vaccine, while data for another similar vaccine showed a slight increase in preterm birth rates.
If the antibody therapy is approved, the FDA says it will continue to monitor the treatment’s safety using multiple data sources. AstraZeneca said it would also conduct regular safety reviews using global data.
What’s next: CDC to review vaccinations for mothers and babies.
If the agency approves the new vaccine, it could be available in the fall — around the same time as Pfizer’s pregnancy RSV vaccine, Abrysvo.
The CDC is expected to advise health care providers on the use of the new treatment later this month. Families and their doctors can then choose a course of treatment that will take into account factors such as time of birth and winter RSV season.
The FDA said there have been no studies on the risks or benefits of vaccinating women against maternal RSV and giving antibodies to their babies.